Adaptive Clinical Systems is an eClinical Technology Solutions Company providing data flow
Integration Optimization Interoperability Innovation

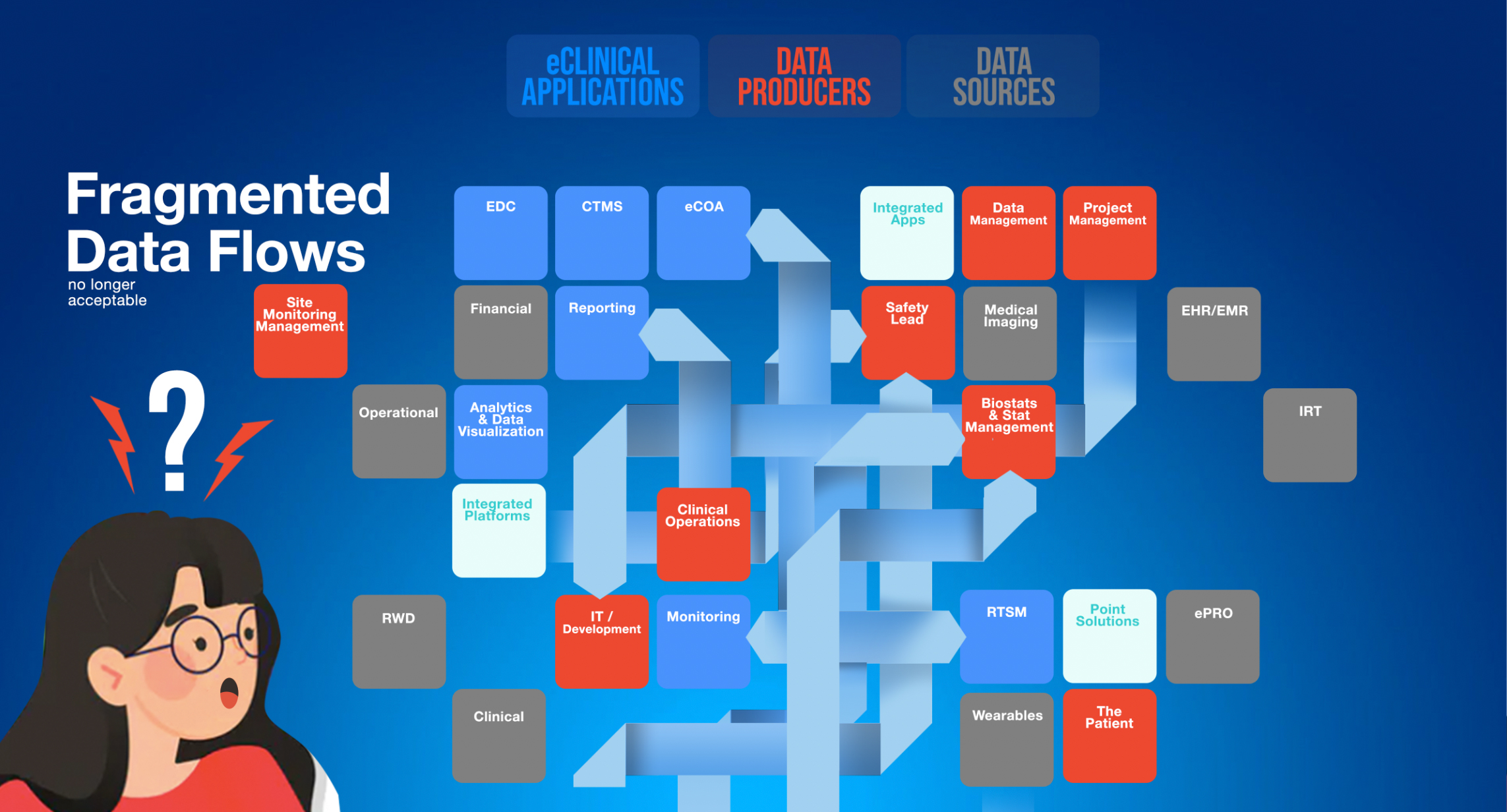
While many partners and eClinical vendors have robust reporting, most do not interoperate. With Adaptive DataVIEW, Sponsors and CROs can incorporate diverse data streams into operational dashboards without the need for convoluted data import and harmonization.

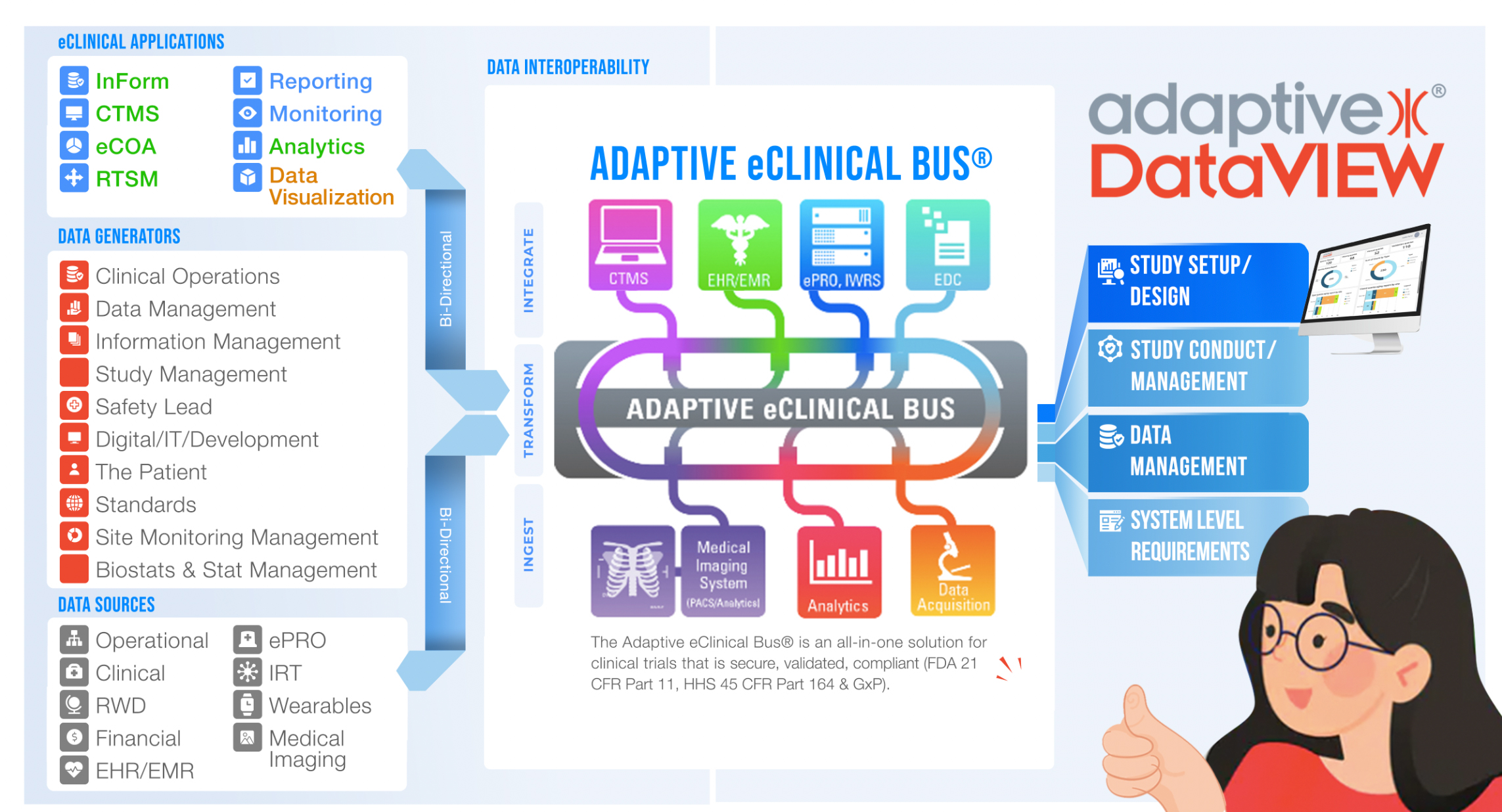
We Modernize Clinical Research with
AI-enabled Interoperability
Adaptive Clinical Systems has launched AI-enabled interoperability for clinical research. With its patent- pending technologies for Automated Data Integration, Data Mapping and FDA-compliant Validation Documentation Generation, Adaptive Clinical adds new automated self-service tools that quickly and efficiently add new data streams from disparate sources.
%
Reduction in Study Setup Time
%
Reduction in Data Management
%
Time Savings
%
Cost Savings



Partners
Adaptive Clinical shares resources to deliver unmatched solutions to our clients.
Experience True Interoperability
Through our innovative Adaptive eClinical Bus® solution, our focus is on helping improve clinical trial operations through interoperability. The Adaptive eClinical Bus® gives our clients the freedom to choose the best eClinical tools of any third-party or proprietary systems while enjoying the benefits of a fully integrated system.
Connect
Freedom to Choose the
Best Clinical Trial Tools
The Adaptive eClinical Bus includes “connectors” for leading clinical trial software from well-known vendors such as Bioclinica, Medidata, and Clinical Conductor; open source clinical trial tools such as OpenClinica and Clinovo; and popular EMR/EHR tools from EPIC, PointClickCare, and others.
Leverage
Leverage Your Proprietary Systems
Leverage your proven, internally-developed and proprietary systems and retain your competitive edge. Adaptive Clinical’s eClinical Bus can easily integrate your technology into an interoperable, efficient, and accurate clinical trials system that streamlines your processes and improves data reliability.
Assemble
Freedom to Choose the Best Clinical Trial Tools
Don’t have tools or don’t want to go to the expense of acquiring them? Adaptive Clinical includes class-leading open source tools that will provide you with all of the functionality you need to easily and quickly get started. Use all of Adaptive Clinical’s tools, or replace some or all of them with your own.
Visualize
Make Your Data
Actionable Faster
As decentralized clinical trials become more complex, Adaptive DataVIEW™ provides holistic access to all your data from internal as well as external (CRO) sources via interactive reporting and visualization. Skip the drill downs, Adaptive DataVIEW has a unique “Single Click-Back” feature direct to the data source.
Meet Ben – Your Data Manager
Don’t have tools or don’t want to go to the expense of acquiring them? Adaptive Clinical includes class-leading open source tools that will provide you with all of the functionality you need to easily and quickly get started. Use all of Adaptive Clinical’s tools, or replace some or all of them with your own.
Blog Posts & Resources
Bridging Clinical Trials with AI: The Effectiveness of Adaptive Tooling and Data Architecture
Bridging Clinical Trials with AIThe Effectiveness of Adaptive Tooling and Data ArchitectureBy Eftim Pop-Lazarov, Chief AI Product Officer, Adaptive...
SCOPE Summit 2024 – Our Take
SCOPE Summit 2024–Our TakeBy Sina Adibi, CEO, Adaptive Clinical SystemsThe show was scheduled in an awkward window on the calendar! Sandwiched...
Normalizing and Harmonizing RWE and EHR with Interoperability in Clinical Trials
Normalizing & Harmonizing RWE & EHR with Interoperability in Clinical Trials Powered by the Adaptive eClinical BusAdaptive Clinical...
















