Decentralized
Clinical Trials
Weaving Together Diverse and Disparate Data Sources
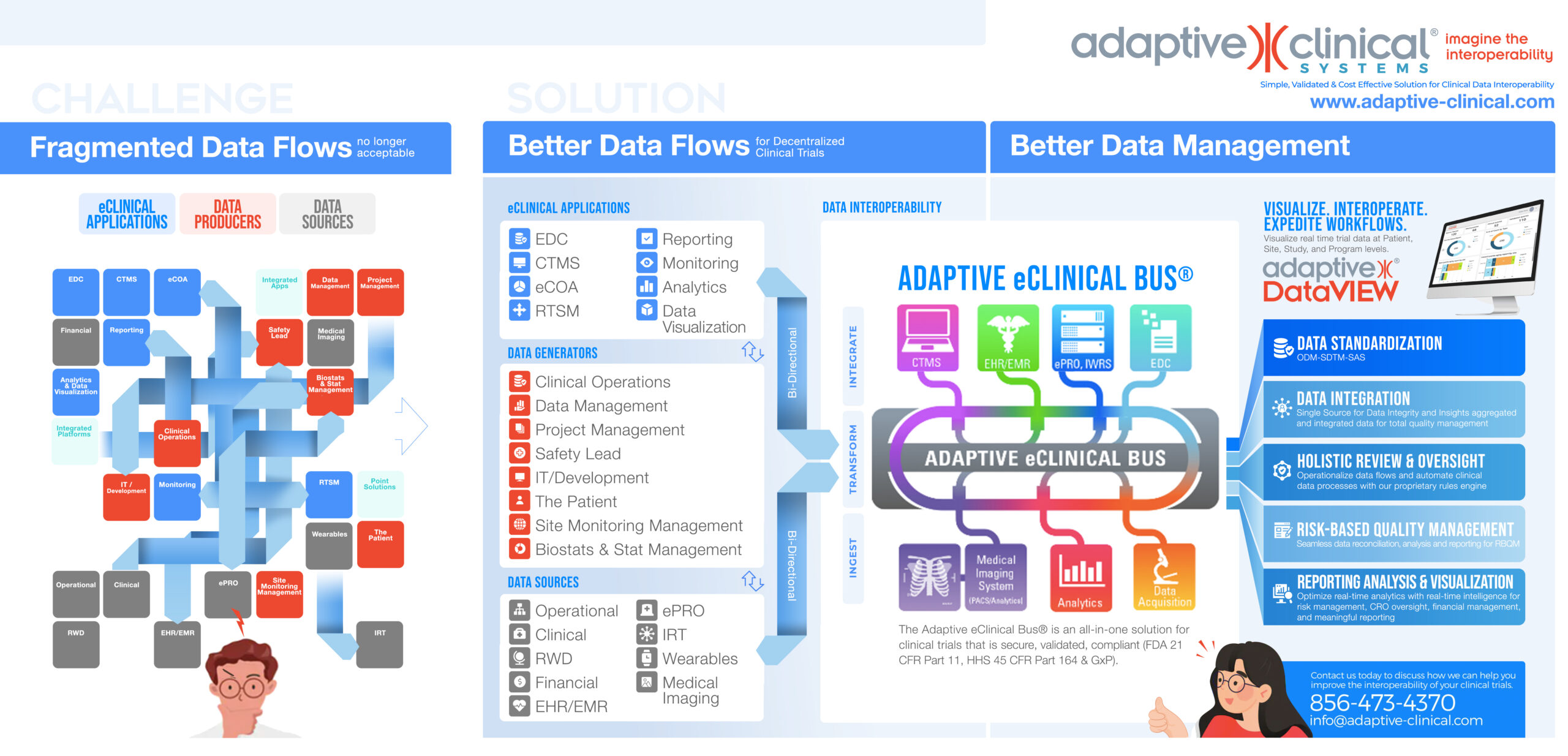
The patient is the source for data as clinical trial operations become increasingly decentralized. This cultural and technological shift has created many new data sources, tied to the patient, and it is imperative to manage and harmonize these new sources. There is also a move to eliminate paper, and bring all eClinical applications on line. Clinical Operations, Data Management, and others find that this migration frequently creates multiple, diverse data and fragmented data flows. In addition, each clinical study creates more data through Data Producers on their team. These Data Producers need access to better data to make data actionable faster.
The imperative to achieve data Interoperability is growing as data streams evolve into a synergistic melding of three perspectives: improved data flow, patient centricity, and emerging technologies.
As noted from the DIA 2021 conference, technologies currently in use in DCTs among healthcare providers continue to expand and trend up from what began as platform solutions to more specialized technologies:
77% |
Mobile technologies(eConsent, EHR, etc.) |
60% |
Wearables |
54% |
In-home devices |
35% |
Sensor |
31% |
AI and ML |
Of the survey responses it becomes clear that the patient is considered the primary beneficiary of the DCT movement.
53% |
Patient convenience |
13% |
Shorter trial timelines |
11% |
More diverse patient populations |
10% |
Reduced cost |