2022 SCOPE CONFERENCE
Summit For Clinical Ops Executives

Adaptive Clinical was pleased to be a Corporate Sponsor of SCOPE 2022. The conference kicked off with some 1,600 professionals from clinical development gathered onsite for this year’s annual meeting in Florida, and another four hundred virtually.
A theme reinforced at the conference is the importance of interoperability to ensure the success of decentralized clinical trials (DCTs) and hybrid trials. Many of the speakers touched on the challenges of DCTs, internal data initiatives, the need for planning as well as the need to use new types of devices to reach under-represented communities. Adaptive Clinical has experience with each of these challenges.
At Adaptive Clinical, we have seen the impact of inadequate upfront planning, and we have great resources, flexibility, and knowledge that we bring to our assignments to help eliminate these challenges. We are an enabler for DCTs. We already partner with leaders in the extensive DCT ecosystem. We understand the difficulty of change. We manage our part of the process, easing the burden on our partners.
Here are some of the highlights that we enjoyed from SCOPE2022:
Challenges of DCTs

The opening, Fireside Chat: Fit-for-Future Data Operations in a Post-Pandemic World, featured speakers from Pfizer, Janssen and Regeneron. This panel shared how the industry accelerated change during the fight against COVID. They shared insights on the importance of flexibility and how the focus on a common enemy, COVID, accelerated change and collaboration. These speakers discussed the importance of pre-planning and the importance of a hard, fast focus to move quickly and efficiently.
Speakers:
► Demetris Zambas | Pfizer
VP and Global Head Data Monitoring and Management
► Darren Weston | Janssen
Sr. Vice President Head of Integrated Data Analytics and Reportingg
► Cynthia Pan | Regeneron
Senior Director Program Operations Leader
In summary, the speakers from this session offered a candid look back on the change that took place within the industry and within their respective companies as we struggled to respond to the COVID-19 pandemic.
Regeneron developed a protocol in only 7 days when they realized one of their drugs had the potential to be a beneficial COVID treatment. Regeneron had to embrace remote options for SDV, when prior to COVID there was much resistance to this. By embracing 100 percent remote monitoring, the company developed close partnerships with CROs and improved integration with them to a true integrated operational structure. Pan said, “I am in awe of the human collaboration at Regeneron. Ownership and accountability were critical for each study.”
Pfizer prepared for the unfamiliar environment and assembled specialty teams. COVID changed paradigms and caused the industry to become more willing to embrace risk, for example around remote monitoring. COVID changed the risk-reward equation and created new internal pressures to move faster. In accordance with their business continuity processes, Pfizer paused some studies and tested their processes. Zambas noted that “this was the ultimate test of processes. The paradigm forced embracing new approaches.”
Janssen worked quickly to become virtual and maintained a clear focus on what is important. The company was able to create change and manage planning virtually. The company changed from 30% remote/70% on site to 70%/30%. At Janssen, the team focused on business continuity, safety and working with global cross functional teams to keep programs going. Weston said, “Collectively sharing all knowledge to make large and small decisions. The Central team challenged to make standards changes and had all key decision makers present to collaborate.”
Operationalizing DCTs
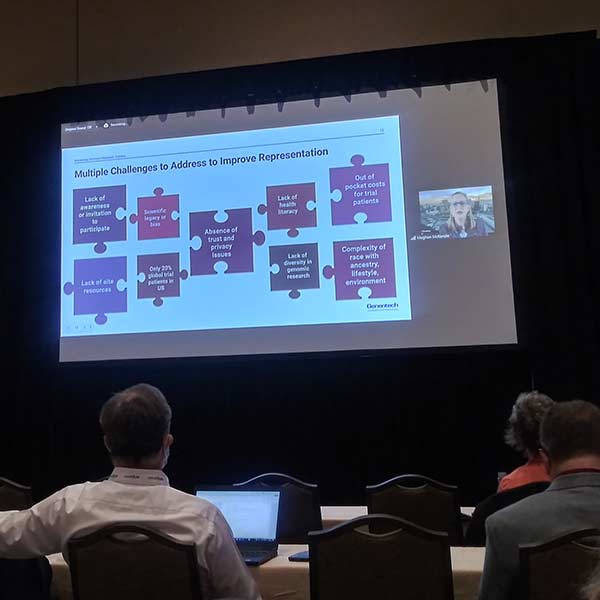
In the session, Practical Approaches for Operationalizing DCTs, Kyle Hogan, EVP Strategy for Datacubed Health, stressed the importance of something we encounter often: the need for extensive planning in all three phases of a trial, including:
► Pre-implementation considerations
► Implementation considerations
► Post-implementation considerations
Hogan stressed the need for flexibility. There will always be outliers and exceptions and there are no one-size-fits-all solutions. Not all patients will be able to provide data the same way. You must have a plan to deal with outliers. Sites need to be prepared to use technology, and issues will arise including things as seemingly simple as passwords. When thinking translations, recognize there will be more need than just standard American English. Again, the key is thorough and detailed upfront planning, with the recognition that you need to build in flexibility.
In the panel discussion, Perspectives on EU MDR – How Companies are Approaching Compliance, the speakers focused on the great work being done in medical devices with EUDAMED and the EU MDR regulatory evolution. Process, lifecycle, and data harmonization will accelerate new and better solutions for patients!
In the presentation, “What is next in DCTs?”, speaker, Joe Dustin, VP Product Strategy at Medable said that he saw the advent of DCTs as the beginning of the next generation platform. Over his tenured career in pharma, he noted that innovation is about change management. It is hard. You cannot prove ROI in the middle of the innovation. It must be a mindset that comes from above and is enforced. It will not just happen. The pharma blockers are the study teams. They are totally focused on their drug, and getting it launched. Dustin recommended that going forward, partner with vendors to develop the new ecosystem.
Recognizing Excellence
The sixth annual Participant Engagement Awards: At this session, nominees with novel and innovative approaches to clinical trial execution present the advancements they’ve developed over the past year. The first-place award was presented to Merck, followed by Guardant, and then to Tryl. Five nominees were chosen from many applicants:
►1. Infiuss Health – serving global representation in patient recruitment throughout the continent of Africa. By creating a pre-enrollment database, they are able to find target populations in 60 minutes with 98% accuracy.
►2. Guardant – helping to expediently match patients with advanced cancers with clinical trials for which they may be suited. With a process that is free to the patients, Guardant’s GAP program can identify 45% of patients needed for complex protocols within one week and follow through with a notification to the patient’s physician.
►3. InVibe – using natural language processing combined with expert analysis, InVibe assists sponsors to develop better communication vehicles in the voice of the patient. They also are developing tools to assess degradation in the patients’ voice, a potential biomarker in categories including Parkinson’s and autism.
►4. Merck – recognizing the need to engage, recruit, and keep children enrolled in pediatric studies, Merck is developing programs using Virtual Reality headsets designed for this purpose. By creating VR patent journeys using this technology, they are also able to access more juvenile populations and be more inclusive in the overall recruitment process.
►5. Tryl – taking a nod from sports consumer loyalty engagement, Tryl is reinventing the wheel in clinical trial patient retention. By applying social proof for intrinsic health as a motivator, Tryl is targeting the patient journey in high dropout therapeutic areas such as dermatology ad oncology.

For more information on our lastest learnings regarding Interoperability, RWD, Remote Patient Monitoring and Electronic Health Records, check out these recent programs:
Check out our most recent blog on Creating Interoperability in Clinical Trials.
Check out latest white paper about incorporating EHR into clinical trials.
See our Roadmap to Interoperability.
See our latest Webinar on RWD and RWE!




