DIA2022
The FUTURE OF HEALTHCARE
If the COVID vaccine is an indication, as an industry we ARE able to improve trial success rates and come up with therapies faster through data sharing, collaboration and regulatory oversight.

The DIA Keynote focused on The Future of Healthcare, and Adaptive Clinical’s CEO Sina Adibi points out the importance of Data in all areas of clinical trials as depicted in this artistic mural created during the conference. At Adaptive Clinical Systems, we understand the importance of data interoperability in clinical trials in order to gain a 360° view of the study and, more importantly, of the patient.
And, just like that, we’re back! The Drug Information Association Global Annual Meeting (DIA) was held June 19-23, 2022, in Chicago after two years of virtual sessions. Adaptive Clinical Systems was pleased to be a sponsor and exhibitor at the event and enjoyed networking with industry leaders. We appreciate the efforts that DIA made to make the conference a safe and positive experience for industry. We feel the momentum created over the last two years and believe positive change will continue to impact the future of clinical trials. The theme for this year’s conference, “The Future of Healthcare,” crystalized for us the industry-wide push to extend the momentum created over the last two years. We feel the momentum and believe positive change will continue to impact the future of clinical trials. Kudos to the DIA, the conference chairs, and the event planners for their efforts in capturing this spirit.

The Patient is the Source
As the world emerges from the SARS-CoV-2 pandemic, DIA focused on “the importance of inspiration and seeing a future where all people have access to lifesaving interventions has never been greater, and DIA aimed to highlight some of the most forward-looking and positive views of what the future of healthcare will be.” The opening keynote session included perspectives on Inclusivity, Global Reach, the Patient, and the importance of digital technologies in extending and improving the patient experience.
The presentation by Ken Getz, Director, Center for the Study of Drug Development at Tufts University, The Future of Healthcare is Centered on the Individual Patient, really struck a chord with us.
Getz noted in his presentation that patients have always been at the center of their care, but now there is growing need and acceptance in clinical R&D that patients be treated as stakeholders and partners. He also focused on the importance of patient preference. What matters to patients and gathering feedback through the clinical development pipeline is an important element of improving study quality and ultimately impacting accessibility, perceptions, and market penetration.
In his presentation, Getz focused on three key drivers for better patient outcomes and noted that the engine for the future of healthcare hinges on:
● Personalized Medicine. Requiring a deep understanding of genetics and combining that with informatics. Reinforcing the importance of properly managing data.
● Patient Engagement. In 2018 we had 100 trials with patient centric characteristics and in 2020 we had 450. This included things like involving the patient in protocol design as well as reporting to individual patients as participants.
● Huge focus on Digital Tech and Data Analytics. Number of data points in pivotal trials has expanded from 494,000 in 2005, to 929,000 in 2010 to 3.5 million in 2020.

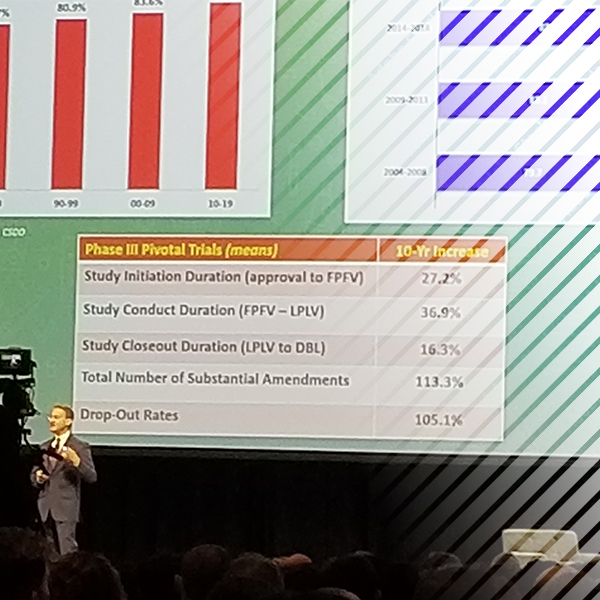
At Adaptive Clinical, we agree with Getz that trials are getting far, far more complex and are taking longer than before. At the same time, headwinds such as patient dropout rates are increasing significantly. This chart from the presentation highlights key study indicators and the importance of these milestones and timelines for submitting new drugs for approval. At each of these phases, Adaptive Clinical has a key driver to help ensure data interoperability and thus speed drugs to approval.
At Adaptive Clinical Systems, we understand the importance of data interoperability in clinical trials in order to gain a 360° view of the study and, more importantly, of the patient. For the last two years, our focus has been on enabling decentralized clinical trials, and properly aggregating clinical data to enable patient-centric trials. There are technology barriers when dealing with – big data – namely velocity, volume, variety and veracity. There is also “value,” the fifth “V,” in data, and the data scientist has to take a deeper dive and find how to decode the data and turn it to insights.
Getz noted in his presentation that even with these advancements – at the same time, trial failure rates are remaining the same – 78% in the 1980s to 88% from 2010-2019. But what is promising is that over the last 20 months, the industry saw that collaboration works. The COVID vaccine was approved for emergency use in a compressed timeframe of 20 months, where in the past the average for vaccine trials was 74 months. The main reasons for this were collaboration, data sharing, best practices and partnerships across pharma, health systems and regulatory. Our challenge now is to make those reforms in process and execution of trials durable and sustainable.
He mentioned some of the shortcomings of trials that have been lurking in the shadows for some time are being addressed. Industry has awakened and continues to work to improve diversity. The Pharmaceutical Industry understands the importance of diversity now more than ever. Industry cannot continue to have statistics that show 70% of trial patients are white as physiological differences between races and gender are real. Today, only 20% to 25% of cardiac study patients are women, and they have an equal chance of heart disease as men.
The Future of Healthcare raises the Importance of Data
As you look at the DIA artists’ mural regarding the Future of Healthcare, Adaptive Clinical agrees with many of the key points from the Keynote, and we want to emphasize that data impacts all areas of the approval process for new clinical trials. Getz went on to close his presentation with these thoughts:
eClinical vendors, CROs and Sponsors are taking notice of the importance of proper data harvesting and curation. Judging from the increased level of interest in interoperability the fragmentation of data is definitely seen as a hindrance. Some are pursuing it as internal projects particularly for organizations that have the budget and talent and others are relying on eClinical vendors to solve the problem.

Adaptive Clinical Systems CEO, Sina Adibi, on the exhibit show floor during DIA2022.
Adopt the
Roadmap to Universal Interoperability
If the COVID vaccine is an indication, as an industry we ARE able to improve trial success rates and come up with therapies faster through data sharing, collaboration and regulatory oversight. As we have said before, something good always comes from something bad. Our key challenge – as noted by Getz – is to make those habits that helped with the speedy COVID vaccine become durable and above all sustainable.
This has to happen now since we must maintain the momentum and make these positive changes permanent if we are to breakout of the current slow state of research. It will take all players in the industry (Vendor, Sponsor, Regulatory) to do this.
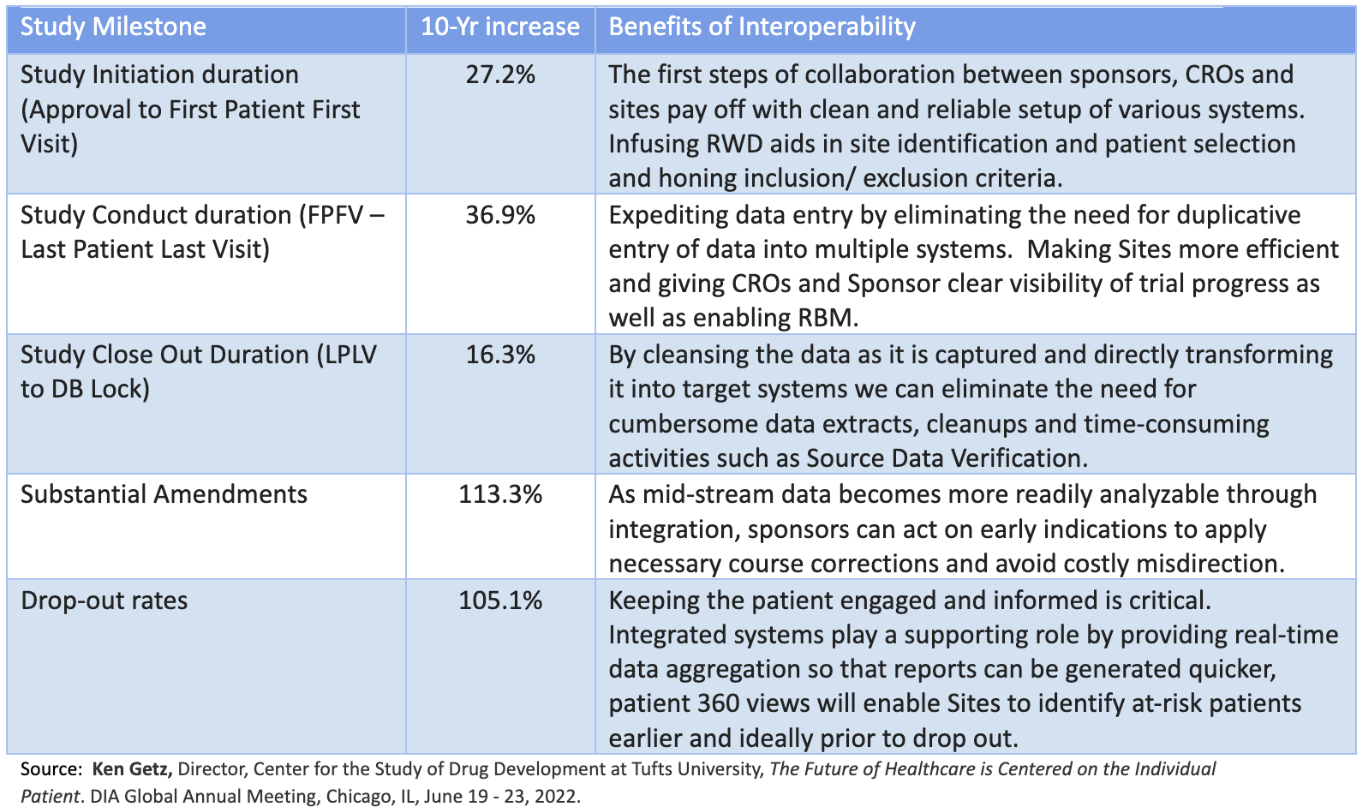
Data Interoperability plays a key role through the study. With the increases in the volume of data throughout a trial, the right data partner plays a critical role in study Milestones. We’ve adapted Getz’s slide to further highlight the benefits of interoperability in clinical trials.
Thank you, DIA, for another interesting year, and for helping shape the coming year. We look forward to DIA 2023 in Boston.
Ask for a demonstration today.
Blog Posts & Resources
Bridging Clinical Trials with AI: The Effectiveness of Adaptive Tooling and Data Architecture
Bridging Clinical Trials with AIThe Effectiveness of Adaptive Tooling and Data ArchitectureBy Eftim Pop-Lazarov, Chief AI Product Officer, Adaptive...
SCOPE Summit 2024 – Our Take
SCOPE Summit 2024–Our TakeBy Sina Adibi, CEO, Adaptive Clinical SystemsThe show was scheduled in an awkward window on the calendar! Sandwiched...
Normalizing and Harmonizing RWE and EHR with Interoperability in Clinical Trials
Normalizing & Harmonizing RWE & EHR with Interoperability in Clinical Trials Powered by the Adaptive eClinical BusAdaptive Clinical...





