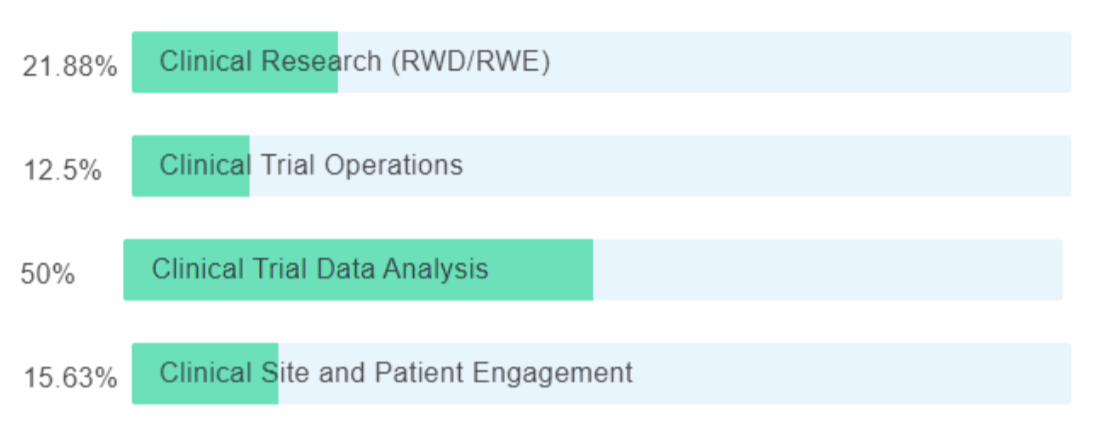
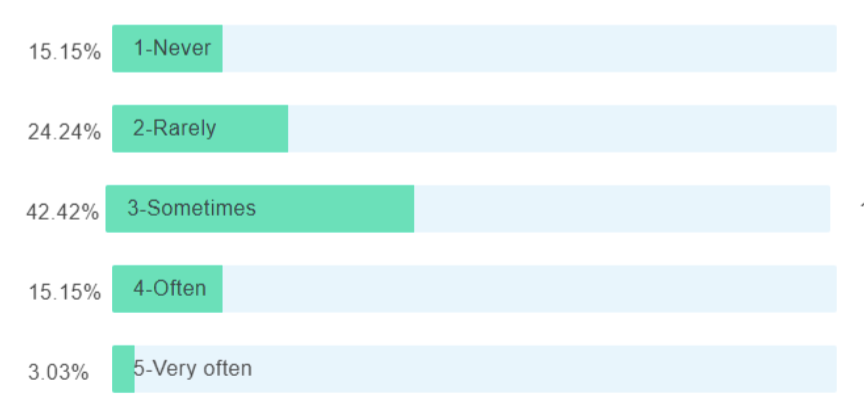
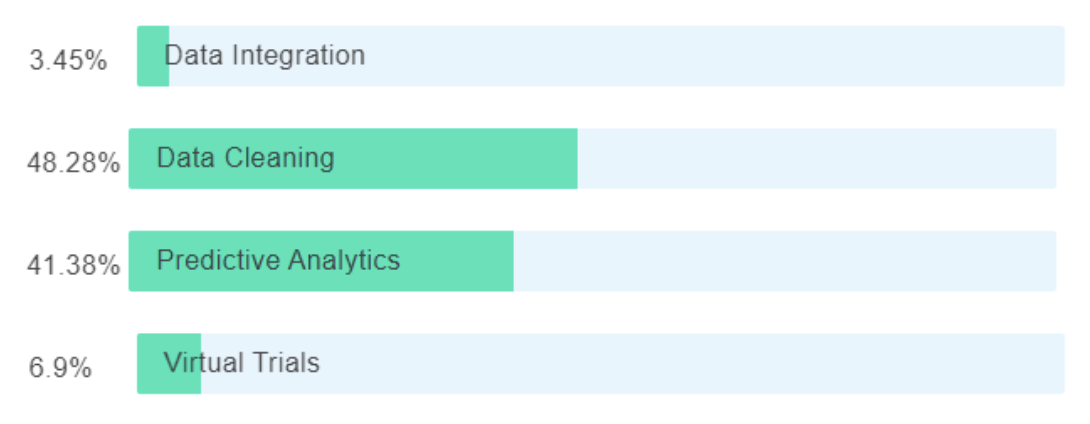
Can AI be used to standardize, harmonize, and partially tokenize the data?
The answer is yes! This is an effective use for smart data mapping. The domain for this will be important. The AI predictions will be better if you can limit the domain. For example, if your intent is medical history for conmed, which is a pain point for data aggregation, be sure and select a training set that ingests drug information data from reliable sources and not open web. There are good models and bad models for every challenge. You need to think through your domain and carefully select the training data and algorithm.
How welcoming are the regulatory bodies with the use of AI in any aspect across the clinical trial phase?
The regulatory bodies are getting there, and they are trying to help and clarify how to use AI. They move slowly. Their focus remains what is referred to as “Responsible AI,” defined as: Safe & Reliable; Trustworthy; Explainable Ethical & Reliable. We interpret that as their intent to make sure the data and the tools are fit-for-purpose. In clinical trials, this is key for any technology, including AI.
What obstacles do you anticipate in using AI derived data for regulatory submissions?
The safe use of Artificial Intelligence is key. Delegating to a black box creates risk. Users must understand for what purpose the models are used. There is a challenge of ethics and bias. If you only feed data of one group of patients, you could have bias. Many AI engines that provide diagnosis were trained on US-based patients, and do not include patients from Europe, Africa, and Asia. Fit-for-purpose in compliance is important. Don’t try to boil the ocean. Pick out what you need and apply the models to those.
When will AI assisted platforms show sponsors a positive ROI?
We are thinking of a singular AI implementation that will take a trial from beginning to end cheaper and faster – that may happen someday, but it is far. Instead, you should look at applications of AI in Analytics or Operations, for example, and identify tasks that are repetitive in nature or very labor intensive such as DM, Biostats or Medical Writing. For example, a very impressive AI application in Medical Writing is when AI converts tables or data into prose and narratives. Except that by using AI, you avoid the laborious implementation and maintenance of Rule Based engines. More importantly, the algorithm refines itself over time if you construct an appropriate feedback loop. Of course, you will always need the Human-in-the-loop. Another way is through computer vision AI which reduces the need for in- person visits to a clinic. This has been deployed in medication adherence and digital biomarker technologies to date and these applications are growing in capability.
With all the AI solutions being developed, what do you think the workforce should do to be ready to use the solutions?
The Greek philosopher Heraclitus is credited with the idea that the only constant in life is change. In the same way that the tech/internet revolution at the turn of the century changed how we live, work and play, so will AI. Bear in mind that AI will not replace humans, but humans using AI will replace other humans in the workforce.