
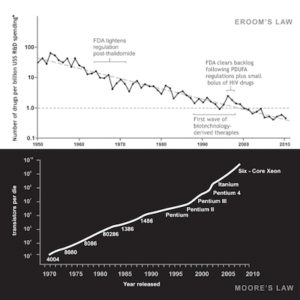
CTIS was abuzz about Digital Biomarkers and their applications in clinical trials. Several definitions floated around, but we liked the simplest one — “user-generated physiological indications captured by a device.” Many of the speakers discussed wearables, but Digital Biomarkers go well beyond that. For example, there is a pill with a heat sensor that can detect whether it has been ingested or not and reports its location in the digestive tract. A perfect example of how technology can help reduce non-compliance by remote patients in a typical clinical trial. Overall, speakers observed that technology is enabling us to capture health data reliably and in real-time. They went further to say that by identifying appropriate Digital Biomarkers as reported by these devices we will be able to overcome some of the longstanding challenges to clinical trials.

 Intuitively bringing the trial to the patient rather than bringing the patient to the trial makes for better and easier interaction with the patient and therefore improved retention and compliance. Furthermore, having “live” telemetry of the patient’s physiological indications prevents chances of “missing” an episodic event that does not repeat in the study or, in worst case, does so after drug launch.
Intuitively bringing the trial to the patient rather than bringing the patient to the trial makes for better and easier interaction with the patient and therefore improved retention and compliance. Furthermore, having “live” telemetry of the patient’s physiological indications prevents chances of “missing” an episodic event that does not repeat in the study or, in worst case, does so after drug launch. If we are to realize the true benefits of wearables, med devices and analytics we need to first create an environment that offers 360° interoperability of all the available data that not only allows us to pull the raw data, but that also allows us to take back the insights and conclusions as determined by the analytics or AI back into the data flow. This information flow modernization is a must and a required data infrastructure investment for any CRO with aspirations of surviving this arms race.
If we are to realize the true benefits of wearables, med devices and analytics we need to first create an environment that offers 360° interoperability of all the available data that not only allows us to pull the raw data, but that also allows us to take back the insights and conclusions as determined by the analytics or AI back into the data flow. This information flow modernization is a must and a required data infrastructure investment for any CRO with aspirations of surviving this arms race.