ELECTRONIC MEDICAL RECORDS
Gain Interoperability by incorporating Electronic Medical Records Data
Quickly and Efficiently using the Adaptive eClinical Bus®
Roughly three-quarters of all Public Health Information can be found in electronic medical records (EMR) managed by Epic Systems Corporation and Cerner Corporation.
Quickly identify subjects that meet study inclusion/exclusion criteria

Directly import demographic information
Easily map routine visit data into eCRF’s
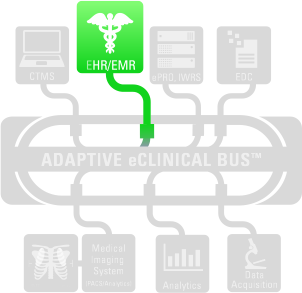
CAPTURING DATA
When Sponsors and CROs capture data from electronic medical records (EMR) and electronic health records (EHR) it’s a win-win – for sponsors, CROs and patients. Not only does it reduce costs and improve efficiencies in clinical trials, it adds a new dimension to the patient profile and allows both health professionals treating patients as well as those overseeing clinical trials to have a more complete picture of the patient’s health. The drive to reduce the cost and complexity of trials is leading to a heightened awareness for a better understanding of patient conditions, more specific therapeutic targeting, improved use of EMRs and electronic technologies for identification of patients and ascertainment of events. EHR may include a patient’s medical history, diagnoses, treatment plans, immunization dates, allergies, radiology images, pharmacy records, and laboratory and test results.
BI-DIRECTIONAL DATA WORKFLOWS
As you look to incorporate EMR data into decentralized trials, Adaptive Clinical’s platform facilitates bi-directional data workflows across all study partners and eClinical data sources. The FDA1 defines interoperability as “the ability of two or more systems or components to exchange information and to use the information that has been exchanged.” The FDA also notes that there are benefits to clinical investigations, patients and other health care providers when interoperability and automated electronic exchange of information exists between the EHR and the sponsor’s electronic systems. The interoperability between EHRs and EDC systems may simplify data collection for a clinical investigation by enabling clinical investigators and study personnel to capture source data at the time of a patient’s point-of-care visit.
INTEROPERABILITY SOLUTIONS
The Adaptive eClinical Bus® solution integrates clinical study data from multiple systems and platforms — EDC, eCOA, CTMS, Medical Imaging, IRT, wearables and analytics/data visualization systems. By incorporating Adaptive DataVIEWTM, sponsors and CROs will obtain a comprehensive data visualization solution that complements and extends any data source from the Adaptive eClinical Bus® to operationalize, gain insight from, exchange and visualize data more quickly. For example, data elements originating in an EHR (e.g., demographics, vital signs, past medical history, past surgical history, social history, medications, adverse reactions) can automatically populate the eCRFs within an EDC system. Use of Surrogate Markers (lowering blood pressure, reducing cholesterol) or use of imaging technologies or biochemical biomarkers can help create a more complete picture and provide better investigative data.
For more information on the benefits of adding EMR to your clinical trials, please click here.