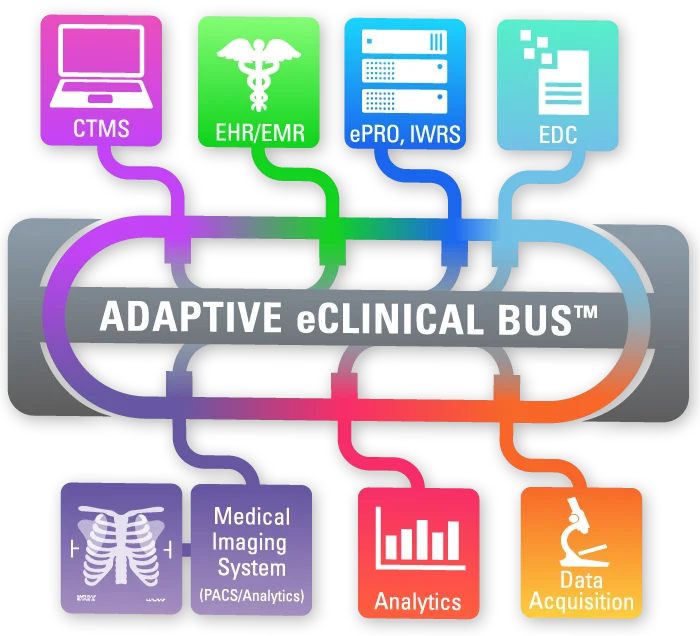
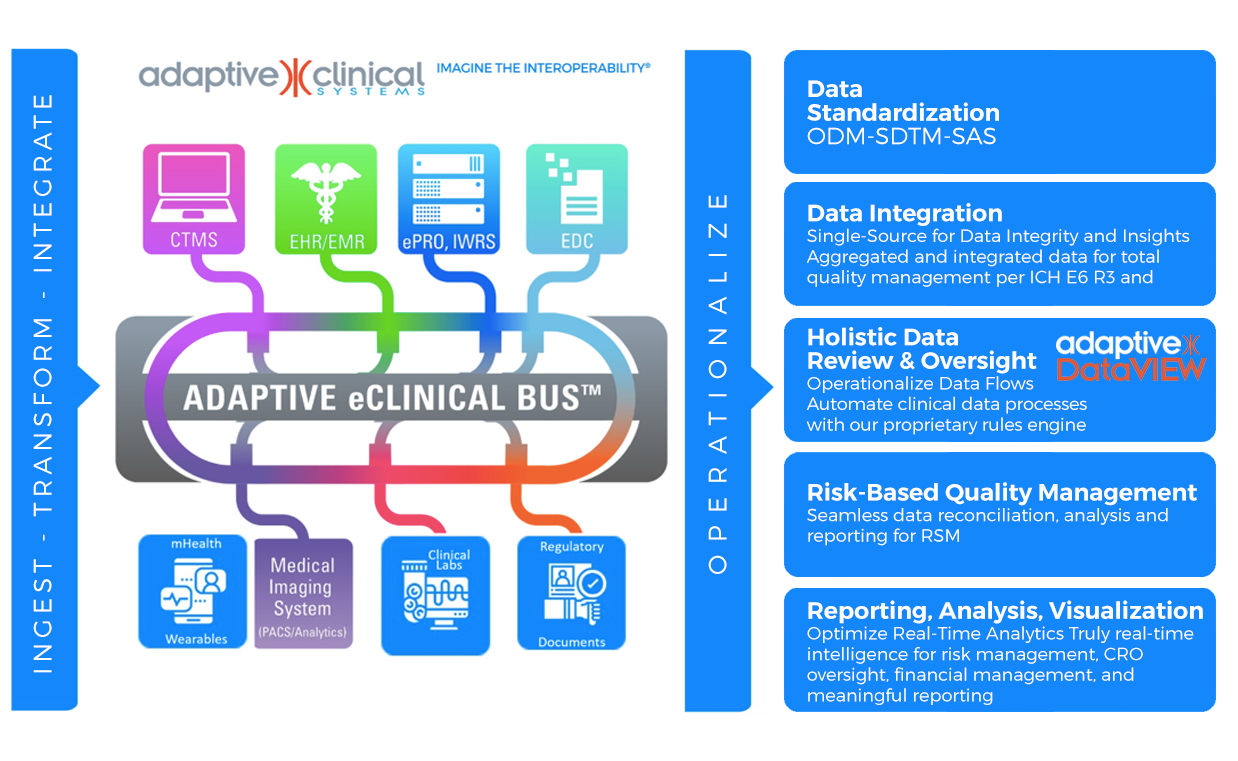
ADAPTIVE eCLINICAL BUS®
At the forefront of AI in clinical research transformation is Adaptive Clinical Systems. Adaptive Clinical’s trailblazing technology addresses the critical challenge of data interoperability in clinical trial operations. The Adaptive eClinical Bus® offers clients the flexibility to select the optimal eClinical tools from third-party and proprietary systems while reaping the benefits of a fully integrated system. This flexibility in tool selection is a watershed moment for numerous life science organizations, enabling them to utilize their proven, internally developed and proprietary systems while retaining their competitive advantage. This saves CROs and Sponsors both time and money in terms of data entry, data verification, data harmonization and aggregated data visualization, and protects their often-substantial investment in legacy systems and data.
Integration Optimization Interoperability Innovation
Simplified Workflow
Adaptive Rules Engine
Adaptive Connectors
Take eSource to the Next Level
Seamless Web & Voice Interface
Reduce Data Management Overhead
Imaging Integration
Leverage Real-time Analytics
Direct Data Capture
The Adaptive eClinical Bus now harnesses AI-enabled interoperability with patent-pending technologies for Automated Data Integration, Data Mapping and FDA-compliant Validation Documentation Generation. The automated platform comes equipped with reusable SaaS components and study templates for quick installation – at the study, site or enterprise level. This unique innovation guarantees privacy and validation quality, setting it apart from conventional generative AI applications that are susceptible to the “neural hallucination” problem. A key feature of Adaptive Clinical’s solution is its integration of generative AI advantages with robust privacy safeguards. Moreover, it emphasizes self-service and automation, allowing users to have control over their data and processes while reaping the benefits of advanced AI technologies. This approach ensures that customers’ data is never incorporated or leaked into the model, harnessing all the benefits of sophisticated AI systems, without compromising privacy or risking accidental data sharing. The Adaptive eClinical Bus is fully compliant with FDA CFR 21 Part 11, HHS 45 CFR Part 164, GxP and other regulatory guidelines.
Adaptive Clinical Systems has been named as a Top eClinical Trial Management Solution Provider by PharmaTech Outlook. See the story here.
Our software helps:
Eliminate duplication of data by capturing and transmitting electronic source data

Encourage entering source data at the point of care and reduce transcription errors

Facilitate remote monitoring of data to reduce the number of onsite visits
Improve site monitoring to minimize the need for cross-reference data in multiple sources

Make it easier for investigators to conduct clinical research
Facilitate the inspection and reconstruction of clinical investigations by FDA

