
Learning More About Electronic Health Records
Adaptive Clinical Systems features leaders from Optum’s Digital Research Network in two webinars. Our
most recent webinar provides an overview of Optum’s recent observational study, Using Electronic
Health Records & Time-saving Technologies to Modernize Clinical Trials.
Wednesday, October 13, 2021
Panelists:
Sina Adibi | President and CEO, Adaptive Clinical Systems
Panelists:
Peter Payne | Head of Digital Research Network, Optum
Cynthia Senerchia | Vice President Data Management and Analytics Digital Research, Optum
When we began this journey we held a webinar from October 2020, Let’s Disrupt eClinical Trials: How Optum is Changing the Industry, and it highlighted new tools and programs that Optum is using to improve patient recruitment. In this second in series where we are sharing our learnings, we surveyed our audience with the same questions to see how our users needs may have changed from when we began this journey until now. And, what a difference a year makes in terms of attendees using EHR/EMR/RWD!
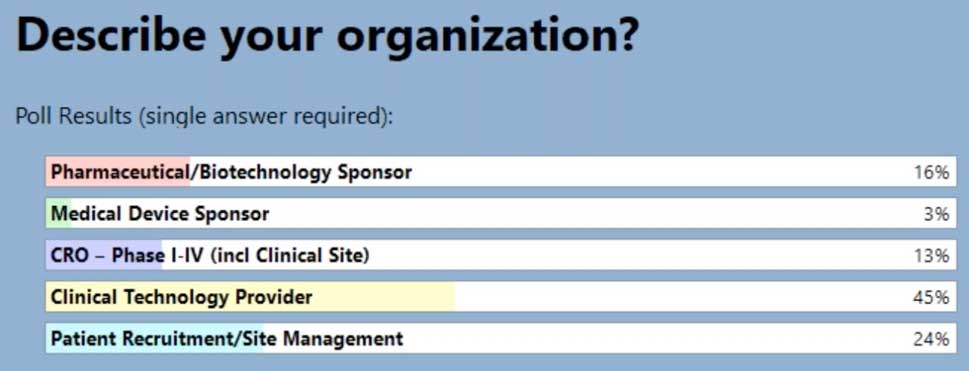
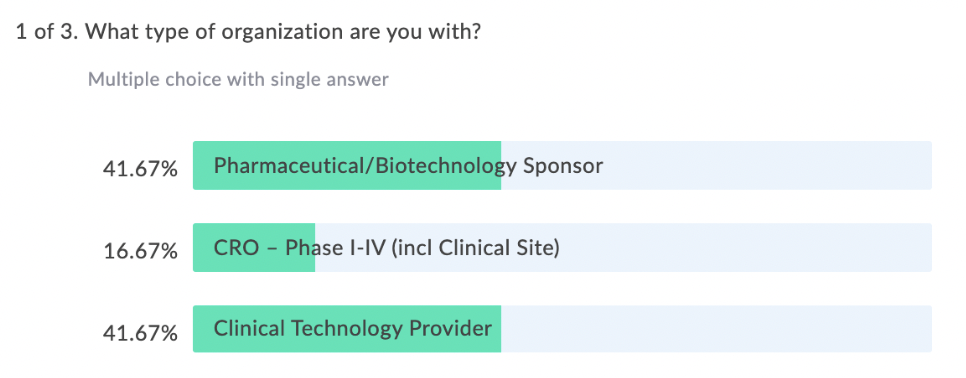

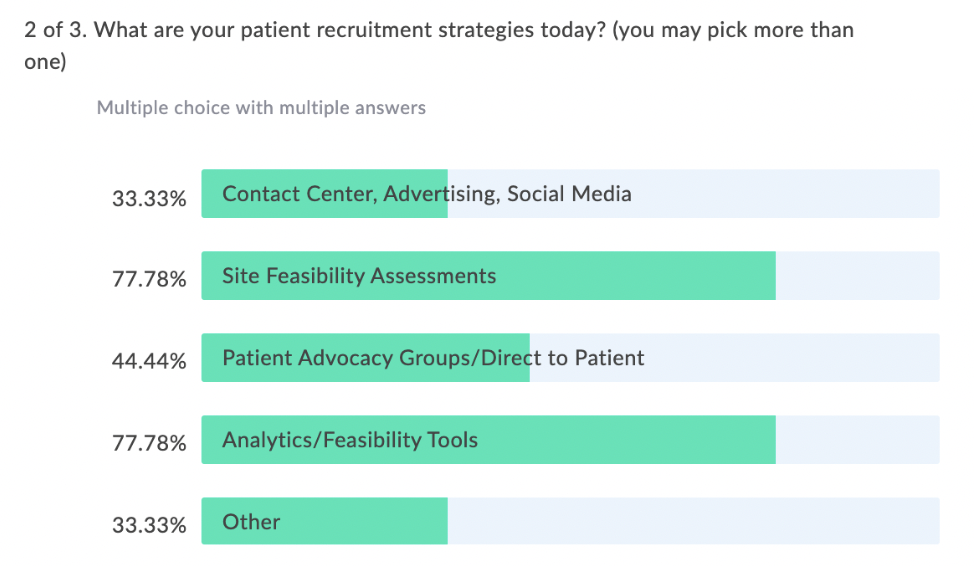
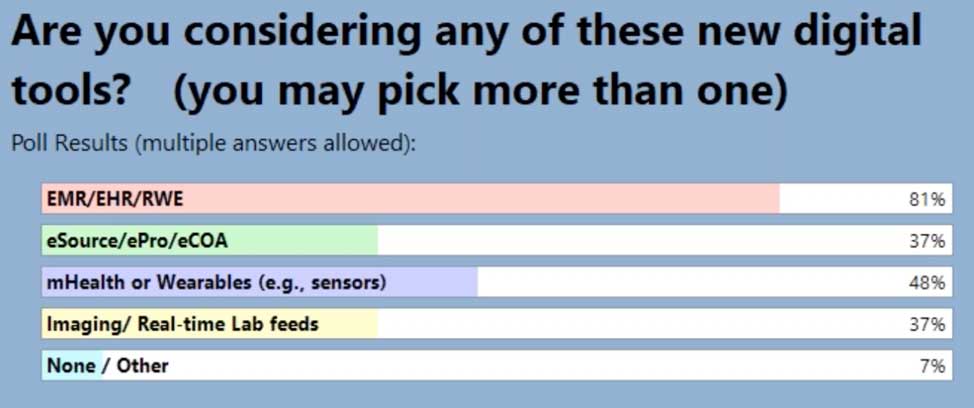
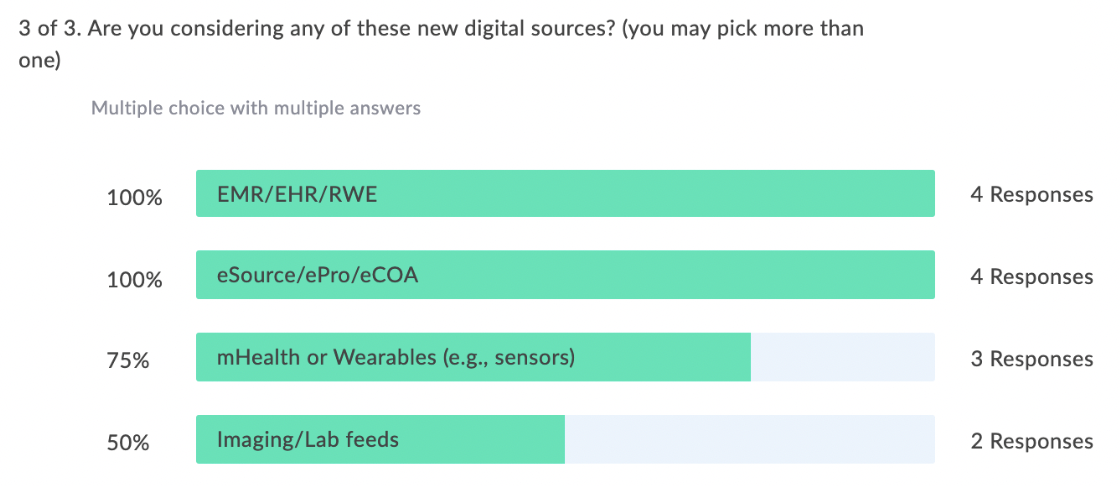
In our latest webinar, Adaptive Clinical Systems’ and Optum’s leadership from the Digital Research Network showcase how Optum is modernizing eClinical Trials. Digitally enabled clinical trials are needed to facilitate faster enrollment and simplify data management to reduce the burden on research staff. Sponsors and CROs need to recruit patients more efficiently, and research sites need better tools to find patients that qualify for trials and simplify data entry for studies. Optum is changing how sponsors and sites work together to streamline the recruitment and data management process. During this webinar Optum’s leadership discusses the processes they have implemented to reduce prescreening efforts and simplify electronic data transfer from eSources to EDC which resulted in clean data and very satisfied research staff.
Other highlights from the observational study include comments on extraction and loading of RWD:
67% of respondents indicated that the process of extracting data directly from the EHR and loading it to the EDC saved them >50% effort of which half indicated it saved them >75%
83% respondents indicated high or extremely high level of confidence in the accuracy and completeness of the EHR data collected during the study
100% of respondents indicated moderate to extremely high level of certainty in using a similar data collection and management procedure for an interventional trial in the future
It is unique that we had eConsent eligibility confirmation coming in from one source, ePRO coming in from someplace else, and three different EHR systems streaming in… Being able to consolidate all of this data and load it into the EDC with Adaptive Clinical every night was so much easier for the sites. Rather them worrying about entering the data into the EDC the next day. – Cynthia Senerchia
Data collection and the query process was very easy and streamlined. In a normal study those tasks take a lot of time and effort. If I were to estimate that time saved, I would say it’s 80% reduction in what we would usually do. Very simple. Very quick. Very efficient. Very timely. —Site 300, Study Coordinator
To access the replay of the most recent webinar, Using Electronic Health Records & Time-saving Technologies to Modernize Clinical Trials. click here: https://adaptive-clinical.com/learning-more-ehr/
To learn more about changing patient recruitment strategies for hybrid and digital trials, see the webinar Let’s Disrupt eClinical Trials: How Optum is Changing the Industry. https://adaptive-clinical.com/2020-webinar3-optum/
