[vc_row type=”full_width_background” bg_position=”left top” bg_repeat=”no-repeat” bg_color=”#04a2c7″ scene_position=”center” text_color=”dark” text_align=”left” top_padding=”0″ bottom_padding=”0″][vc_column][divider line_type=”No Line”][/vc_column][/vc_row][vc_row type=”in_container” bg_position=”left top” bg_repeat=”no-repeat” scene_position=”center” text_color=”dark” text_align=”left” top_padding=”30″][vc_column width=”1/1″][vc_column_text]
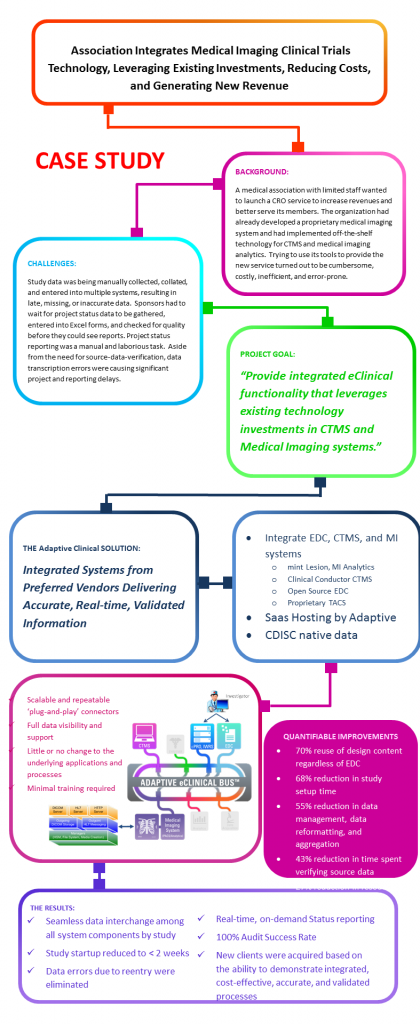
CASE STUDY
A medical association with limited staff wanted to launch a CRO service to increase revenues and
better serve its members. The organization had already developed a proprietary medical imaging system and had implemented off-the-shelf technology for CTMS and medical imaging analytics. Trying to use its tools to provide the new service turned out to be cumbersome, costly, inefficient, and error-prone.
Study data was being manually collected, collated, and entered into multiple systems, resulting in late, missing, or inaccurate data. Sponsors had to wait for project status data to be gathered, entered into Excel forms, and checked for quality before they could see reports. Project status reporting was a manual and laborious task. Aside from the need for source-data-verification, data transcription errors were causing significant project and reporting delays.
The Adaptive Clinical Solution
Using the Adaptive eClinical Bus® to create an integrated system that leveraged the investments in technology from preferred vendors, the association was able to provide a fast, accurate, real-time offering to its members. The newly integrated system provided scalability, full data visibility, and required little or no change to existing applications or automated processes. Manual data manipulation was virtually eliminated, and there was little training required.
The Result
The resultant improvements in the system’s speed, automation, quality, and timeliness of data enabled the association to quickly generate revenue from several new studies. The scalable and repeatable automation provided full data visibility and greatly improved the process. Study startup time was reduced to less than 2 weeks, data errors caused by reentry were eliminated, and the client passed every Sponsor audit without issue.[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column width=”1/1″][divider line_type=”No Line”][/vc_column][/vc_row][vc_row][vc_column width=”1/1″][/vc_column][/vc_row]