Enabling Decentralized Clinical Trials with Seamless, Remote Integration of Devices &
eClinical Systems
Original Panel Discussion Webinar took place, August 3, 2021 11:00 AM
Presenters:
Rich Murg | Global Vice President Business Development, Software Solutions, Bioclinica
Sina Adibi | President and CEO, Adaptive Clinical Systems
Jennifer Jackson | Director for Connectivity, Masimo
COVID-19 has accelerated the adoption of decentralized trials, which often requires the remote capture of patient vital signs at specific time points. To do this efficiently and have instant access to the data, the direct transfer of data from remote measurement technologies to eClinical systems is required.
To show how this can be accomplished, representatives from Masimo, Adaptive Clinical Systems, and Bioclinica will demonstrate the direct connection capabilities established between the Masimo’s Rad-97 for blood pressure monitoring and Bioclinica EDC via the Adaptive eClinical Bus®.
In this webinar, these industry leaders discuss the challenges facing the addition of remote patient monitoring (RPM) to decentralized clinical trials.
“When incorporating remote patient monitoring devices, the device must match the workflow” — Jennifer Jackson
“Find the way to eliminate the paper and the excel worksheets” – Sina Adibi
“Develop solutions that are easily repeatable” – Rich Murg
“Biggest recurring challenge – Resistance to Change” – Rich Murg
“Automate the data flows. Enable your data managers to become data scientists and not data verification staff.” – Sina Adibi
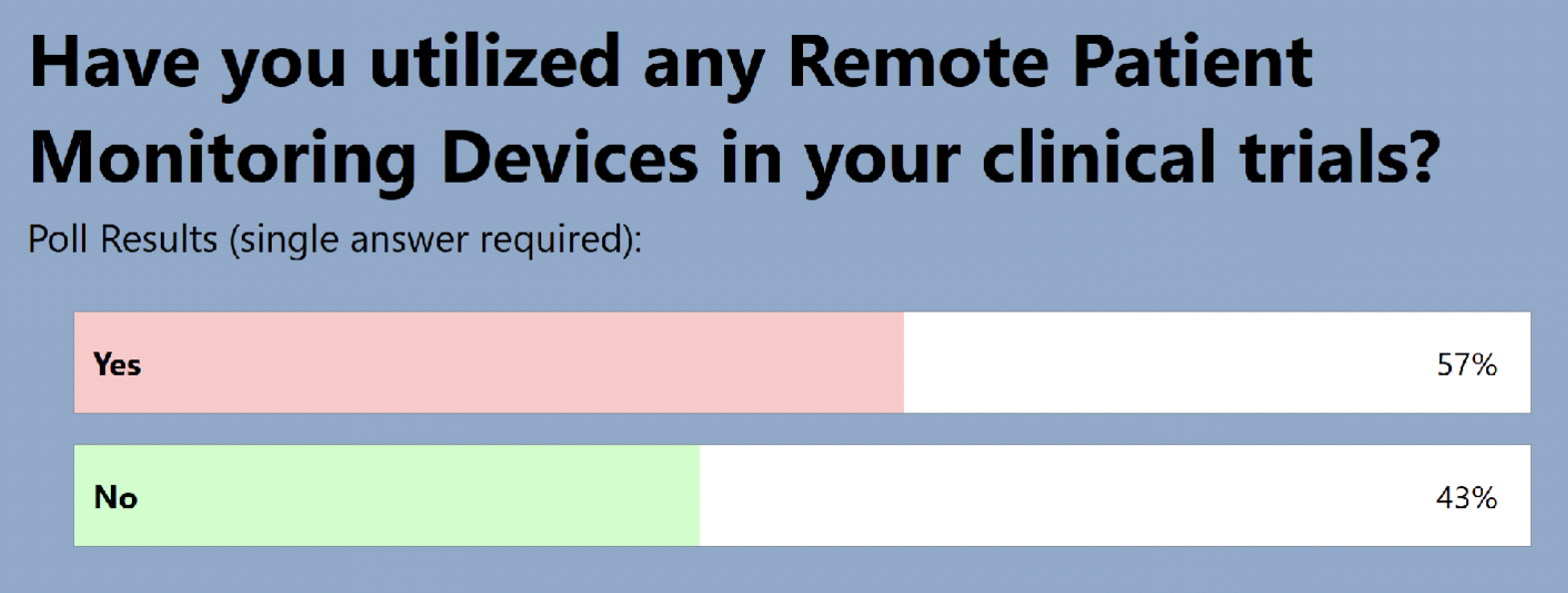
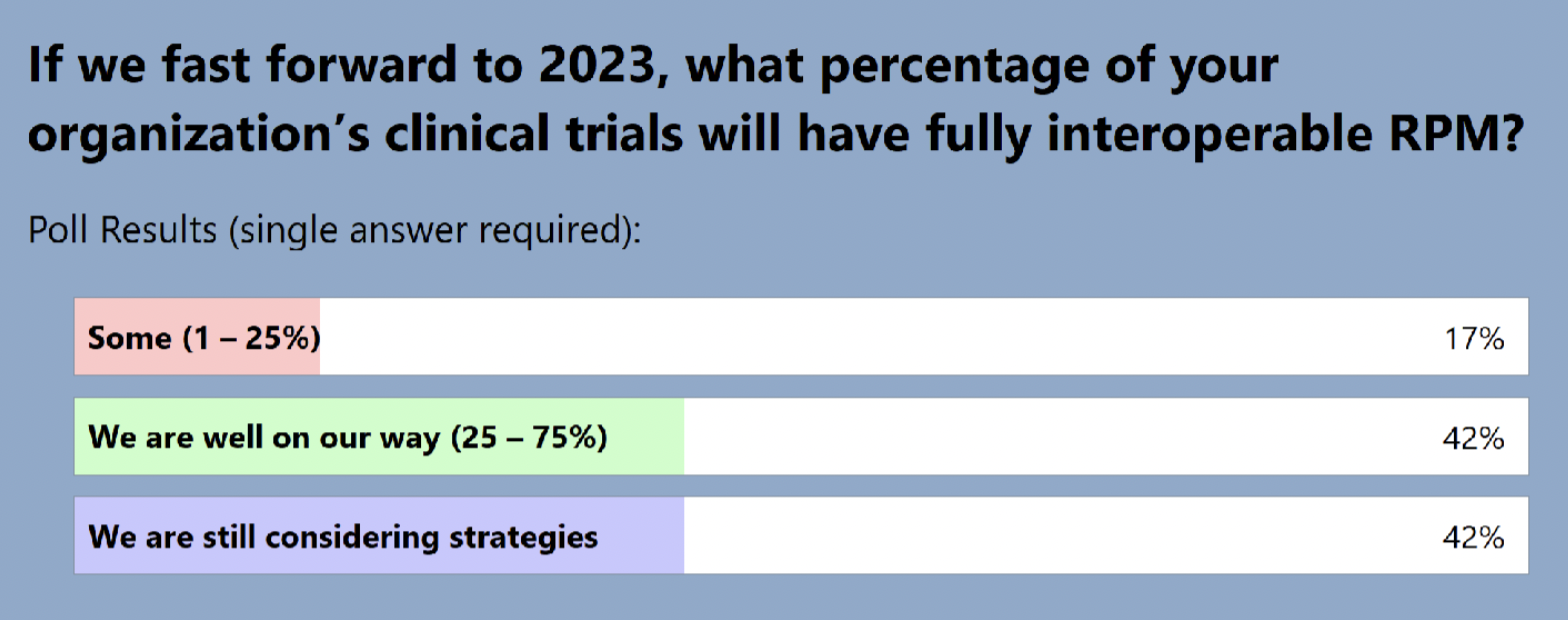
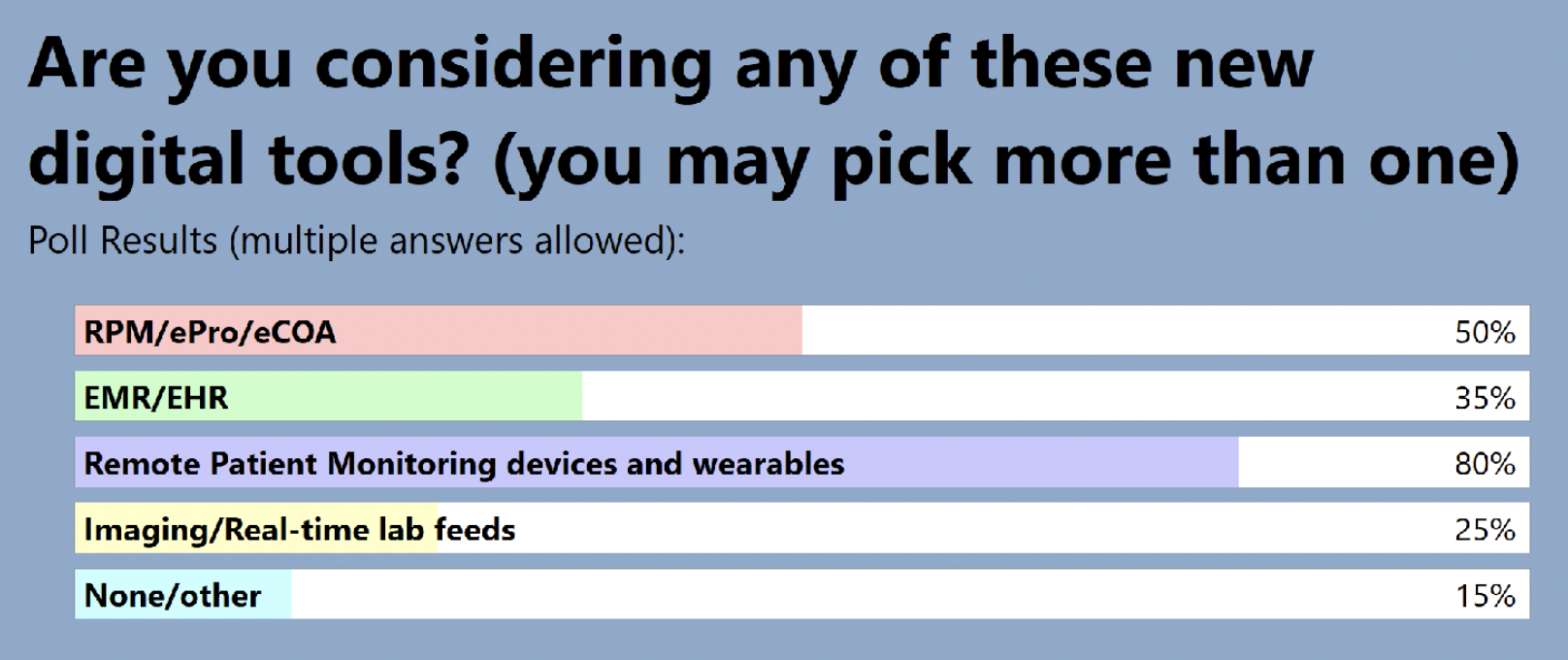
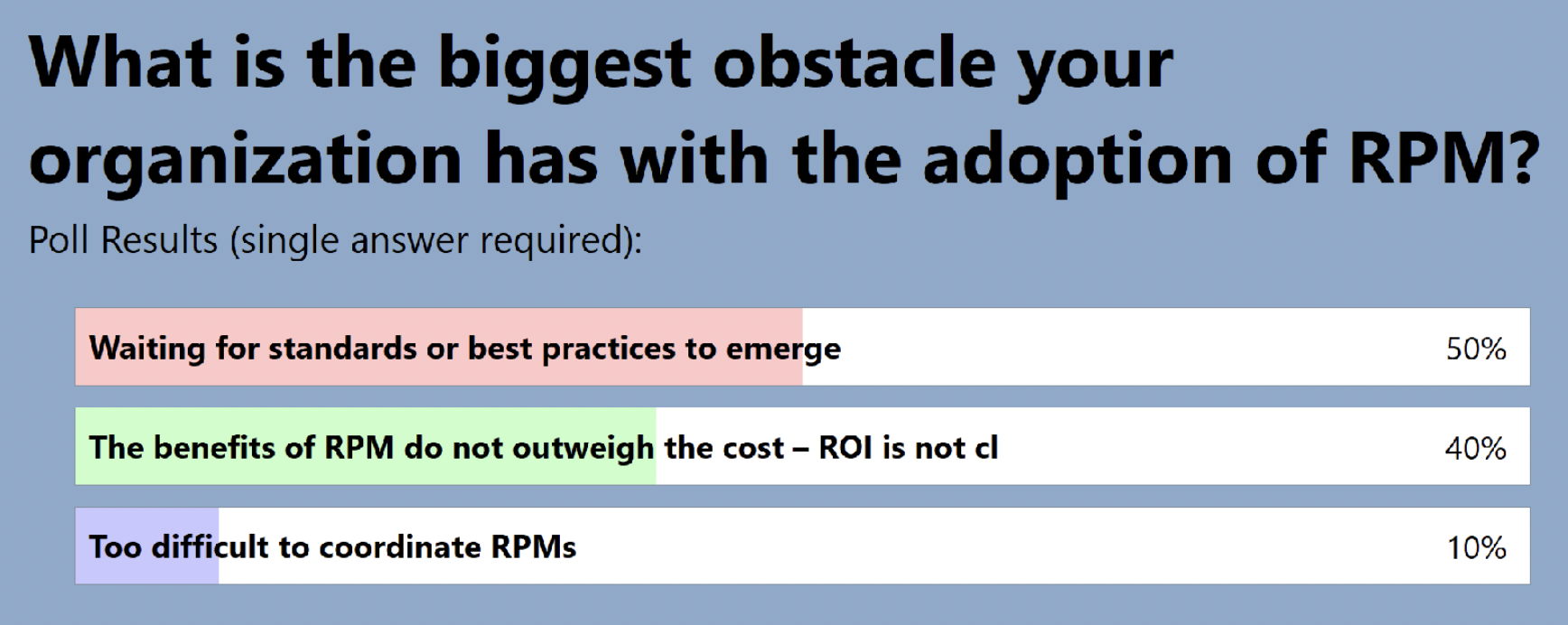
The audience also shared where they are in terms of adding RPM to clinical trials: